Carbon nanotube@layered nickel silicate coaxial nanocables as excellent anode materials for lithium and sodium storage†
Abstract
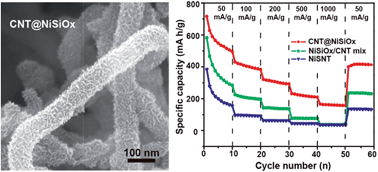
Layered nickel silicate provides massive interlayer space similar to graphite for the insertion and extraction of lithium ions and sodium ions; however, the poor electrical conductivity limits its electrochemical applications in energy storage devices. Herein, carbon nanotube@layered nickel silicate (CNT@NiSiOx) coaxial nanocables with flexible nickel silicate nanosheets grown on conductive carbon nanotubes (CNTs) are synthesized by a mild hydrothermal method. CNTs serve as conductive cables to improve the electron transfer performance of nickel silicate nanosheets, resulting in reduced contact and charge-transfer resistances. In addition to a high specific surface area, short ion diffusion distance and good electrical conductivity, one-dimensional coaxial nanocables have a stable structure to sustain volume change and avoid structure destruction during the charge–discharge process. As an anode material for lithium storage, the first cycle charge capacity of the CNT@NiSiOx nanocables reaches 770 mA h g−1 with the first cycle coulombic efficiency as high as 71.5%. Even after 50 cycles, the charge capacity still reaches 489 mA h g−1 at a current density of 50 mA g−1, which is nearly 87% and 360% higher than those of the NiSiOx/CNT mixture and nickel silicate nanotube, respectively. As anode materials for sodium storage, the coaxial nanocables exhibit a high initial charge capacity of 576 mA h g−1, which even retains 213 mA h g−1 at 20 mA g−1 after 16 cycles.

 Please wait while we load your content...
Please wait while we load your content...