Co-electrolysis of H2O and CO2 in a solid oxide electrolysis cell with hierarchically structured porous electrodes†
Abstract
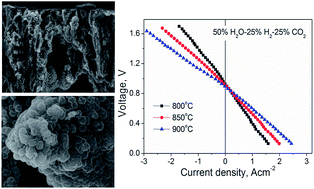
A solid oxide electrolysis cell with novel asymmetric-porous structured electrodes has been fabricated by the combination of freeze-drying tape-casting and impregnation methods. The electrodes possess unique channel-like pores with nano- or sub-micron-sized catalysts homogeneously coated on the inner face of the porous scaffold. The straight channel-like pores in the electrodes facilitate mass transport while the nano- or sub-micron-sized catalysts promote the electrode electrochemical reactions. The cell demonstrates low electrode polarization resistance values of 0.27, 0.19 and 0.14 Ω cm2 at OCV in 50 vol% H2O–25 vol% H2–25 vol% CO2 at 800, 850 and 950 °C, respectively. The cell DC voltage–current density dependence is generally linear at all temperatures, and high current densities of 0.8, 1.1 and 1.66 A cm−2 for 50 vol% H2O–25 vol% H2–25 vol% CO2 co-electrolysis have been obtained with 1.30 V applied voltage at 800, 850 and 900 °C, respectively. Compared to that of the cell fabricated by conventional or phase-inversion methods, the mass transportation limitation phenomenon in the porous electrodes is mitigated and cell performance is greatly improved.

 Please wait while we load your content...
Please wait while we load your content...