Oligothiophene-bridged perylene diimide dimers for fullerene-free polymer solar cells: effect of bridge length
Abstract
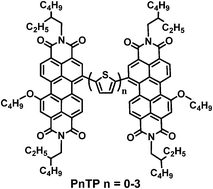
A series of perylene diimide (PDI) dimers (PnTP, n = 0–3) with oligothiophenes as bridges were designed, theoretically calculated, synthesized, and developed as electron acceptors for polymer solar cells (PSCs). The effects of oligothiophene bridge length on the absorption, energy level, charge transport, morphology and photovoltaic properties of the molecules were investigated. These molecules exhibited good thermal stability with decomposition temperatures of 367–413 °C, broad and strong absorption in the visible region (400–700 nm), and appropriate HOMO (−5.74 to −5.61 eV) and LUMO (−3.84 to −3.72 eV) energy levels. When blending these PDI acceptors with a low bandgap polymer donor PBDTTT-C-T, the PSCs exhibited power conversion efficiencies of 0.76–3.61%.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...