Novel visible-light sensitive vanadate photocatalysts for water oxidation: implications from density functional theory calculations†
Abstract
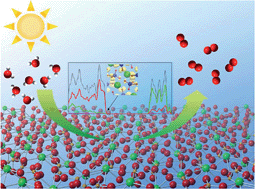
Two vanadates, Ag2Sr(VO3)4 and Sr(VO3)2, have been studied as visible-light-driven water oxidation photocatalysts with the help of density-functional theory calculations. Our computational results for the density of states and partial charge densities implied that Ag2Sr(VO3)4 and Sr(VO3)2 possess desirable electronic structures for the water oxidation reaction, i.e., the valence band (VB) maximum of Ag2Sr(VO3)4 consists of multiple orbitals of Ag d and O p, while Sr(VO3)2 has a broad VB associated with oxygen non-bonding states. We have experimentally demonstrated that these vanadates efficiently oxidize water to O2 under irradiation of visible light in the presence of the sacrificial agent.

 Please wait while we load your content...
Please wait while we load your content...