Guanidinium octahydrotriborate: an ionic liquid with high hydrogen storage capacity†
Abstract
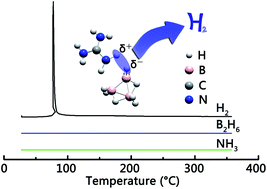
For chemical hydrogen storage, capacity is one key criterion that has spurred intense efforts to investigate compounds with high hydrogen content. The guanidinium cation and the octahydrotriborate anion possess 6 H+ and 8 H−, respectively. The combination of these two ions yields guanidinium octahydrotriborate with 13.8 wt% hydrogen. This paper presents its facile synthesis, as confirmed by 11B and 1H nuclear magnetic resonance spectroscopy. The results show that guanidinium octahydrotriborate is an ionic liquid with a melting point below −10 °C, which makes it a possible injectable/pumpable hydrogen carrier. It decomposes selectively to hydrogen, in stark contrast to the formation of various boranes from related solid octahydrotriborates. The much improved H2 purity can be ascribed to the more effective combination of H+ and H−, and the higher H+/H− ratio in liquid guanidinium octahydrotriborate.

 Please wait while we load your content...
Please wait while we load your content...