A two-dimensionally microporous thiostannate with superior Cs+ and Sr2+ ion-exchange property†
Abstract
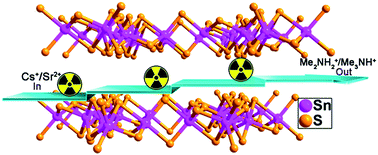
The removal of highly radioactive and long-lived 137Cs+ and 90Sr2+ from solution is of significance for radionuclide remediation. Herein we prepared a two-dimensionally microporous thiostannate, namely (Me2NH2)4/3(Me3NH)2/3Sn3S7·1.25H2O (FJSM-SnS), and systematically investigated its Cs+ and Sr2+ ion-exchange performance in different conditions. The structural stabilities and variation, ion-exchange kinetic and isothermal behavior, pH-dependent distribution coefficients (Kd), ion-exchange in simulated groundwater and ion-exchange applied to chromatography have been investigated. The results indicated that the maximum Cs+ and Sr2+ ion-exchange capacities of FJSM-SnS were 408.91 mg g−1 and 65.19 mg g−1, respectively. In particular, FJSM-SnS showed quick ion-exchange ability and wide pH resistance (0.7–12.7) which make it outstanding among the ion-exchangers. An ion-exchange chromatographic column was firstly studied for chalcogenido ion-exchange materials, that is, a column filled with 3.0 g FJSM-SnS could remove 96–99% of Cs+ ion and near 100% of Sr2+ ion at low ionic concentrations in 900 bed volumes solutions. Furthermore, the title material could be synthesized on a large scale by a facile, one-pot and economical solvothermal method. The relatively low cost but remarkable ion-exchange performance makes it promising for radionuclide remediation.

 Please wait while we load your content...
Please wait while we load your content...