High-efficiency and stable quasi-solid-state dye-sensitized solar cell based on low molecular mass organogelator electrolyte†
Abstract
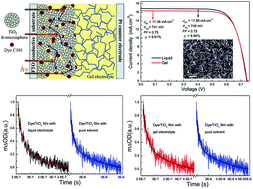
The highest photoelectric conversion efficiency (9.61%) for a quasi-solid-state DSSC (QS-DSSC) based on a low molecular mass organogelator (LMOG) is achieved using N,N′-1,5-pentanediylbis-dodecanamide as a LMOG in conjunction with a TiO2 photoanode of sub-microspheres sensitized with a high-absorptivity Ru complex (C101). The competition of interfacial kinetic processes between the recombination of the dye cations with photoinjected electrons and the regeneration of the dye cations by I− is investigated by transient adsorption measurements. The data revealed that there is an efficient interfacial charge separation at the TiO2 photoelectrode/electrolyte interface and the dye is rapidly regenerated, which contributes to the high photocurrent. Furthermore, using electrochemical impedance spectroscopy (EIS) and controlled intensity modulated photocurrent/photovoltage spectroscopy (IMPS/IMVS), it is determined that the negative shift of TiO2 conduction band edge of the QS-DSSC contributes to the high Voc. Moreover, the QS-DSSC exhibits significantly improved stability during the accelerated thermal and light-soaking test. During the accelerated aging test, there is almost no change in the short-circuit current density (Jsc) in the QS-DSSC, while the Jsc of the liquid electrolyte based DSSC decreases sharply. These results are very important for the application and commercialization of DSSCs.

 Please wait while we load your content...
Please wait while we load your content...