Development of a nanoporous adsorbent for the removal of health-hazardous fluoride ions from aqueous systems†
Abstract
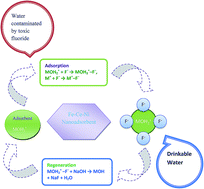
A new crystalline and hybrid Fe–Ce–Ni nanoporous adsorbent was developed, and tested to establish its efficiency, using kinetic and thermodynamic studies, for fluoride removal. The adsorption properties of the developed adsorbent were studied using batch and column methods and the noticeable fluoride adsorption capacity was 285.7 mg g−1. The pH range for the maximum removal of fluoride on the metal oxide adsorbent surface was found to be 5.0–7.0. The adsorption kinetics fitted well with a pseudo-second-order as compared to a pseudo-first-order rate expression. Herein, four models are used to fit the experimental data to explain the adsorption isotherm. The adsorption isotherm data fitted rationally well into both Freundlich and Dubinin–Radushkevich (D–R) isotherm models. Thermodynamic examination demonstrated that fluoride adsorption on the Fe–Ce–Ni nanoadsorbent was reasonably spontaneous and endothermic. Most commonly existing anions, such as Cl−, NO3−, SO42−, CO32−, and HCO3−, but not PO43−, showed no significant counter ion effect on fluoride adsorption efficiency. The adsorbent was easily regenerated up to 95% with an alkali solution. The capability to revive and reuse the nanoadsorbent makes it a smart sustainable material.


 Please wait while we load your content...
Please wait while we load your content...