Photocatalytic reactivity of {121} and {211} facets of brookite TiO2 crystals†
Abstract
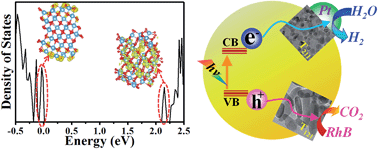
For the facet engineering of brookite TiO2, the surface atomic structure is known but the electronic structure has been rarely studied to date. Herein, we investigated both the surface atomic and electronic structure of brookite TiO2 with various facets exposed. Theoretical calculations reveal that the {121} surface contains more undercoordinated Ti atoms and a higher surface energy than that of the {211} surface, and the experimental results show that brookite TiO2 nanorods exposed with majority {121} facets (T121) have a lower valence band (VB) potential; those above-mentioned superior properties enable T121 to show excellent performance in RhB photodegradation. Nevertheless, the electronic structure analyzed from the Density of State (DOS) plots revealed that the electron density is dispersed in the bulk for TiO2 covered with a {121} surface, indicating that the electrons might be more reluctant to migrate from bulk to surface, which might be the reason for the poor H2 productivity of T121. In contrast, brookite TiO2 nanosheets exposed with dominant {211} facets (T211) exhibited a much higher conduction band (CB) potential resulting in a much higher H2 evolution rate (801 μmol h−1) in photocatalytic water splitting. Accordingly, combining the analyses of the surface atomic structure and electronic band structure, it is suggested that, for brookite TiO2, the {121} surface is beneficial for photocatalytic oxidation reactions while the {211} surface can facilitate the photocatalytic reduction process.

 Please wait while we load your content...
Please wait while we load your content...