Fabrication of YxBi1−xVO4 solid solutions for efficient C2H4 photodegradation†
Abstract
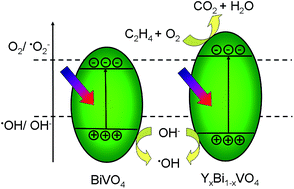
Photocatalytic oxidation of ethylene continues to be a challenge at the frontier of chemistry. Previous investigations have shown that BiVO4 possesses strong photo-oxidative properties which can efficiently oxidize water and decompose organics in aqueous solutions; however, its conduction band minimum is too low to utilize the photo-generated electrons. Herein we report for the first time its effects on gaseous C2H4 photo-oxidation by fabricating YxBi1−xVO4 (x = 0.00–1.00) semiconductors with a polymeric method. Phase analysis identified that there are both a region of monoclinic and tetragonal phase coexistence (0.05 ≤ x ≤ 0.45) and a solid solution region (0.5 ≤ x ≤ 1.0). UV-visible diffusive reflectance spectra and Mott–Schottky analysis revealed that the incorporation of Y3+ widened the band gap by shifting upward the conduction band minimum as well as shifting downward the valance band maximum. Remarkable C2H4 photodegradation was obtained upon the Y0.85Bi0.15VO4 photocatalyst and its superior performance is ascribed to the synergistic effect of C2H4 adsorption, active oxygen species ˙O2− utilization, and relatively large surface area. The present study will be valuable for further investigation on both BiVO4 and hydrocarbons (HC) degradation.

 Please wait while we load your content...
Please wait while we load your content...