Ammonia borane modified zirconium borohydride octaammoniate with enhanced dehydrogenation properties†
Abstract
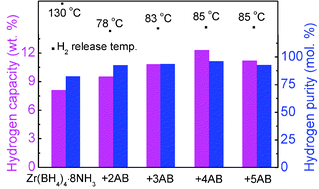
A new complex system, Zr(BH4)4·8NH3–nNH3BH3 (n = 2, 3, 4, 5), was prepared via ball milling of Zr(BH4)4·8NH3 and NH3BH3 (AB). The combination strategy effectively suppressed ammonia release and reduced the dehydrogenation temperature when compared to the individual compounds. In the optimized composition, Zr(BH4)4·8NH3–4AB, the hydrogen purity was improved to 96.1 mol% and 7.0 wt% of hydrogen was released at 100 °C. These remarkable improvements are attributed to the interaction between AB and the NH3 group in Zr(BH4)4·8NH3, which enables a more active interaction of Hδ+⋯−δH. These advanced dehydrogenation properties suggest that Zr(BH4)4·8NH3–4AB is a promising candidate for potential hydrogen storage applications.

 Please wait while we load your content...
Please wait while we load your content...