Is there a universal reaction mechanism of Li insertion into oxidic spinels: a case study using MgFe2O4†
Abstract
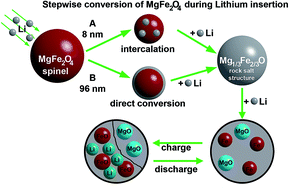
Structural and electronic changes during Li insertion into spinel-type MgFe2O4 particles with different sizes were studied applying a multi-method approach yielding a detailed picture about distinct reaction steps during Li uptake. A small amount of Li is intercalated into the smaller particles (8 nm) at the beginning of the reaction while no such reaction step occurs for the large crystallites (ca. 100 nm). Li uptake is accompanied by a reduction of Fe3+ ions and simultaneous movement from the tetrahedral to an empty octahedral site. After uptake of 2 Li per formula unit all ions have moved from tetrahedral to free octahedral voids resulting in the formation of a disordered NaCl-type material. Insertion of 4 further Li atoms transforms the crystalline material to an amorphous and inhomogeneous product consisting of a Li2O matrix with embedded nanosized metallic Fe and MgO particles. During the charge process Fe is oxidized to FeO and Li2O is converted to Li.

 Please wait while we load your content...
Please wait while we load your content...