Diethylenetriamine (DETA)-assisted anchoring of Co3O4 nanorods on carbon nanotubes as efficient electrocatalysts for the oxygen evolution reaction†
Abstract
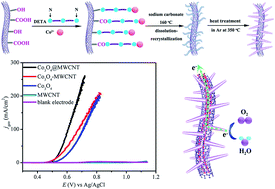
A Co3O4 nanorod–multiwalled carbon nanotube hybrid (Co3O4@MWCNT) has been fabricated as a highly efficient electrocatalyst for water oxidation in alkaline electrolytes. The well-defined Co3O4 nanorods were successfully anchored onto mildly oxidized MWCNTs with the assistance of diethylenetriamine (DETA) following a simple route. The as-prepared hybrid possesses a promising BET specific surface area of about 252 m2 g−1 and shows excellent electrocatalytic activity towards oxygen evolution reactions (OERs) with an onset potential of about 0.47 V vs. Ag/AgCl and an overpotential of 309 mV to achieve a current density of 10 mA cm−2 in 1.0 mol L−1 KOH. In addition, the Co3O4@MWCNT catalyst exhibits prominent stability during long-term electrolysis of water. We attribute the enhanced performance to a synergic effect between MWCNTs and Co3O4 nanorods by gaining insight into the electrochemical properties of the Co3O4@MWCNT hybrid. This work offers a useful way to synthesize one-dimensional (1D) metal oxides combined with 1D carbon materials for wide applications in energy storage and conversion.

 Please wait while we load your content...
Please wait while we load your content...