Heterojunction confinement on the atomic structure evolution of near monolayer core–shell nanocatalysts in redox reactions of a direct methanol fuel cell†
Abstract
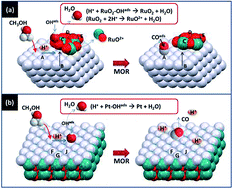
This research demonstrated that the methanol oxidation reaction triggers the protonation corrosion on the multi-walled carbon nanotube supported bimetallic nanocatalysts (NCs) in different geometrical configurations. Such corrosion pathways are confined by the geometrical configuration of the heterogeneous interfaces at the bimetallic oxide nanoparticles. In bimetallic alloyed NCs, the platinum (Pt) and ruthenium (Ru) domains were exposed simultaneously to the redox environment, therefore, the geometrical confinement on the atomic restructure is weak. This leads to substantial dissolution followed by the regrowth of hydrophilic components in redox conditions. For core–shell NCs, the shell crystal would prevent the core from direct contact with the redox environments. The heteroatomic junction forms ordered Pt atomic stacking at the ruthenium oxide crystallite. Such conformation confinement enhanced the Pt to Ru charge donation, thus the CO tolerance factor was also enhanced by more than two orders of magnitude when compared to the module durability of the direct methanol fuel cell compared to that of PtRu alloy.

 Please wait while we load your content...
Please wait while we load your content...