Improved dehydrogenation properties of the LiNH2–LiH system by doping with alkali metal hydroxide†
Abstract
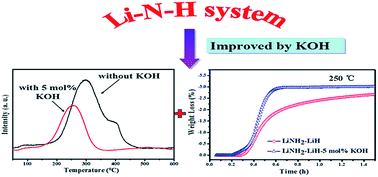
In this study, the hydrogen desorption properties of the LiNH2–LiH system were investigated and discussed by doping with alkali metal hydroxide (LiOH, NaOH, and KOH). It was determined that the three types of hydroxides are effective for enhancing the hydrogen desorption properties of the LiNH2–LiH system, among which, KOH shows the best effect. In comparison with the broad-shaped hydrogen desorption curve of the LiNH2–LiH system without an additive, the hydrogen desorption curve of the LiNH2–LiH–0.05KOH composite becomes narrow. By doping with 5 mol% KOH, the dehydrogenation onset temperature of the LiNH2–LiH composite is decreased by about 36 °C, and the dehydrogenation peak temperature is lowered by about 42 °C. Detailed structural investigations reveal that during ball milling, the doped alkali metal hydroxide can react with LiH to convert to alkali metal hydride, which is responsible for the improvement in the hydrogen desorption properties of the LiNH2–LiH system.

 Please wait while we load your content...
Please wait while we load your content...