Highly reduced VOx nanotube cathode materials with ultra-high capacity for magnesium ion batteries†
Abstract
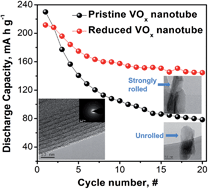
Here, we describe novel VOx nanotubes with vanadium at various oxidation states (V3+/V4+/V5+) as cathode materials for magnesium ion batteries. The VOx nanotubes synthesized by a microwave-assisted hydrothermal process using an amine as an organic template show a high initial discharge capacity (∼218 mA h g−1) of more than 200 mA h g−1 and an outstanding cycling performance, which have not been previously reported for magnesium ion batteries. These improvements in the electrochemical performance of our VOx nanotubes originate from the trivalent vanadium ions generated in the highly reduced VOx nanotubes. The VOx nanotubes with trivalent vanadium ions exhibit a lower charge transfer resistance at the electrode/electrolyte interfaces and superior cycling performance than the VOx nanotubes containing vanadium ions of a higher oxidation state. We first suggest that the pristine oxidation state of the vanadium ions and the maintenance of a bonding structure on the surface of the VOx nanotubes are the most important factors determining the magnesium insertion/extraction kinetics into/out of the VOx nanotubes. Our findings offer a breakthrough strategy for achieving high-energy-density magnesium rechargeable batteries using VOx nanotube cathode materials in combination with nanoarchitecture tailoring.

 Please wait while we load your content...
Please wait while we load your content...