Optical detection of submicromolar levels of nitro explosives by a submicron sized metal–organic phosphor material†
Abstract
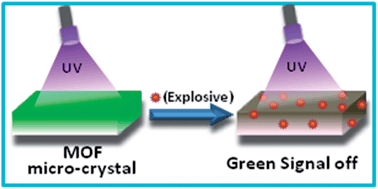
Two isomorphous submicron sized metal–organic network compounds, [Y2(PDA)3(H2O)1]·2H2O (PDA = 1,4-phenylenediacetate), 1 and [Y1.8Tb0.2(PDA)3(H2O)1]·2H2O, Tb@1 have been synthesized by employing solvent assisted liquid grinding followed by heating at 180 °C for 10 min and washing with water. Single crystal X-ray data of bulk 1 confirmed a three dimensional porous structure. The structure and morphology of 1 and Tb@1 were systematically characterized by PXRD, TGA, DSC, IR, SEM and EDX analysis. Dehydrated Tb@1 [Tb@1′] shows a high intense visible green emission upon exposure to UV light. The green emission of Tb@1′ was used for the detection of nitro explosives, such as 2,4,6-trinitrophenol (TNP), 1,3-dinitro benzene (DNB), 2,4-dinitro toluene (DNT), nitro benzene (NB), and 4-nitro toluene (NT) in acetonitrile. The results show that the emission intensity of dehydrated Tb@1′ can be quenched by all the nitro analytes used in the present work. Remarkably, Tb@1′ exhibited a high efficiency for TNP, DNB and DNT detection with KSV [KSV = quenching constant based on linear Stern–Volmer plot] values of 70 920, 44 000 and 35 430 M−1, respectively, which are the highest values amongst known metal–organic materials. Using this material submicromolar level (≡ 0.18 ppm), a detection of nitro explosives has been achieved.

 Please wait while we load your content...
Please wait while we load your content...