A graphene-based electrochemical filter for water purification†
Abstract
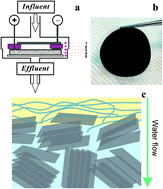
A novel graphene-based electrochemical filter with carbon nanotubes as conductive binders was developed for water purification. Ferrocyanide (Fe(CN)64−) was used as a model compound to study electron transfer mechanisms in the electrochemical filter. A 70% : 30% ratio of graphene : carbon nanotube was optimal for electrochemical oxidation of Fe(CN)64−, and electrooxidation rates increased linearly with increasing concentration of influent Fe(CN)64−. The results of chronoamperometry and normal pulse voltammetry indicated that mass transfer increased up to 15-fold in the electrochemical filter as compared to a batch electrooxidation system. Finally, the efficiency of graphene-based filters for electrooxidation of organic pollutants was evaluated with three selected organic compounds. The oxidation rates increased with increasing anode potential and reached maximum removal rates of 0.010 mol h−1 m−2 (88% removal), 0.064 mol h−1 m−2 (93% removal), and 0.014 mol h−1 m−2 (87% removal) at an applied anode potential of 0.8 V (vs. Ag/AgCl) for tetracycline, phenol, and oxalate, respectively. Overall, the results exemplified the advantages of contaminant removal using a graphene electrode in a flow-through system and demonstrated the potential of using graphene-based electrochemical filters for water purification.

 Please wait while we load your content...
Please wait while we load your content...