Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst
Abstract
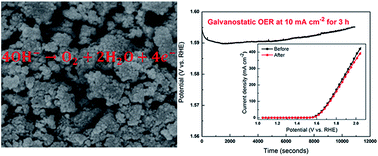
Water splitting, to produce hydrogen and oxygen, has long been considered to be a desirable option for the storage of electrical energy. The catalysts for oxygen evolution reactions (OER) are very important in this process. Herein, we have synthesized Co3V2O8 nanoparticles by a simple and cost-effective technique, which have low crystallinity and large specific surface area (122.8 m2 g−1). Because of the low crystallinity, large specific surface area and suitable pore size, Co3V2O8 nanoparticles yielded an electrocatalytic OER current density of up to 429.7 mA cm−2 at 2.05 V vs. RHE and low OER over potentials of 359 mV (at 10 mA cm−2) and 497 mV (at 100 mA cm−2). In addition, the OER stability of the Co3V2O8 catalyst was very excellent, and the current density at 2.05 V was reduced by just 7.3% after galvanostatic OER measurement at 10 mA cm−2 for 3 h. This work demonstrates that binary metal oxides Co3V2O8 is a highly active and stable oxygen evolution electrocatalyst that can potentially replace expensive noble metal-based anode catalysts for electrochemical water splitting to generate hydrogen fuels.

 Please wait while we load your content...
Please wait while we load your content...