Towards understanding of the mechanism of stepwise zeolite recrystallization into micro/mesoporous materials†
Abstract
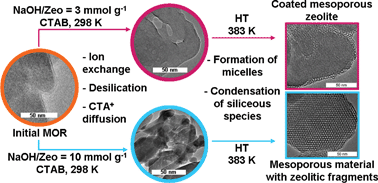
The mechanism of stepwise mordenite recrystallization has been studied by a complex of physicochemical methods: multinuclear MAS NMR, X-ray diffraction (XRD), X-ray fluorescence (XRF), transmission electron microscopy (TEM), thermogravimetric analysis (TGA) and nitrogen adsorption–desorption. The recrystallization procedure included 4 steps: (1) treatment in alkali, (2) treatment in the presence of cetyltrimethylammonium bromide (CTAB) surfactant; (3) hydrothermal treatment and (4) hydrothermal treatment after pH adjustment to 8. Intermediate products were recovered during each reaction step and thoroughly investigated. The results pointed to the dissolution/re-assembling mechanism. The comparison with one-step recrystallization discussed previously suggests that the stepwise addition of NaOH and the surfactant does not affect significantly the recrystallization mechanism. In contrast, stepwise hydrothermal treatment with the intermediate pH regulation enhances the condensation of siliceous species formed during desilication, leads to their rearrangement into an ordered mesoporous phase and results in the product yields of 100%. Depending on the degree of zeolite dissolution and the extent of mesoporous phase overgrowth, two different types of materials were obtained: coated mesoporous zeolites and mesoporous materials containing small zeolitic fragments in the walls. It was shown that the latter could be obtained only using a stepwise procedure.
- This article is part of the themed collection: Porous Materials (FEZA 2014)

 Please wait while we load your content...
Please wait while we load your content...