Mn and Co co-substituted Fe3O4 nanoparticles on nitrogen-doped reduced graphene oxide for oxygen electrocatalysis in alkaline solution†
Abstract
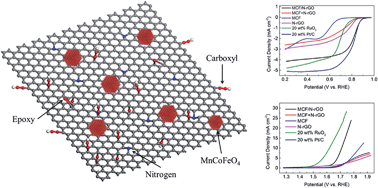
A dispersion of Mn and Co co-substituted Fe3O4 (MCF, Mn : Co : Fe = 1 : 1: 1) nanoparticles on nitrogen-doped reduced graphene oxide (N-rGO) nanosheets was prepared by a hydrothermal method. This catalyst exhibited 80% of the oxygen reduction reaction (ORR) activity of a Sigma 20 wt% Pt/C catalyst; and 61% of the oxygen evolution reaction (OER) activity of a 20 wt% RuO2/C catalyst in alkaline solution. Extensive material characterizations by field-emission transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) analysis, and inductively coupled plasma mass spectrometry (ICP-MS) were undertaken to suggest some possible reasons for the good electrochemical performance. The catalyst also delivered good performance in full zinc–air cell tests where it was used in the air electrode. The MCF catalyst has effectively combined the ORR activity of manganese oxide, the OER activity of cobalt oxide; and the electronic conductivity of bulk Fe3O4 into an integrated bifunctional catalytic system; and its good contact with the N-rGO nanosheets also reduces the external transport resistance in oxygen electrocatalysis.

 Please wait while we load your content...
Please wait while we load your content...