High temperature structural stability, electrical properties and chemical reactivity of NdBaCo2−xMnxO5+δ (0 ≤ x ≤ 2) for use as cathodes in solid oxide fuel cells†
Abstract
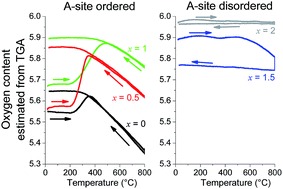
The effects of Mn substitution for Co on the crystal chemistry, oxygen content, thermal expansion and electrical conductivity of the NdBaCo2−xMnxO5+δ perovskites (0 ≤ x ≤ 2) have been investigated. The NdBaCo2−xMnxO5+δ samples exhibit structural changes with increasing Mn contents from orthorhombic (x = 0) to tetragonal (0.5 ≤ x ≤ 1) then to cubic (1.5 ≤ x ≤ 2.0) symmetry. All the samples lose oxygen when heated in air at T > 400 °C although the degree of oxygen loss and kinetics of oxygen exchange between the gas phase and oxide decrease with increasing Mn contents. The thermal expansion coefficients evaluated from ex situ XRD and electrical resistivity decrease with increasing Mn substitution and the values for the x = 1.5 and 2.0 compositions match with those of the Ce0.8Gd0.2O1.95 (GDC) and La0.8Sr0.2Ga0.8Mg0.2O2.8 (LSGM) electrolytes. With electrical conductivity values of >100 S cm−1 at 800 °C and good chemical stability with GDC and LSGM, the Mn-substituted perovskites are promising cathode materials for SOFCs.

 Please wait while we load your content...
Please wait while we load your content...