Thin film fabrication and characterization of proton conducting lanthanum tungstate†
Abstract
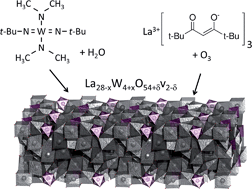
Thin films of the proton conducting lanthanum tungstate phase, La28−xW4+xO54+δv2−δ, were fabricated by atomic layer deposition (ALD) and characterized by impedance spectroscopy. The films were prepared by combining the processes of deposition of La2O3 and WO3 using [La(thd)3 + O3] and [(tBuN)2(Me2N)2W(VI) + H2O]. Deposition of WO3 by the above mentioned precursor combinations is investigated in the range of 250–375 °C proving ALD growth at temperatures 275–325 °C. WOCl4 + H2O was evaluated for WO3 deposition. The observed surface sensitive growth resulted in limited film growth on selected substrates. The process of lanthanum tungstate deposition was optimized with respect to control of stoichiometry and 0.9 and 1.2 μm thick films were deposited on MgO and Pd for cross-plane and in-plane impedance spectroscopy measurements, respectively. Due to short-circuiting of films on Pd after heat treatment, an alternative deposition procedure was devised which reduced short-circuiting significantly. The films show primarily ionic conductivity under wet conditions. Oxide ions are the dominating conductors above 650 °C, while protons become more dominant at lower temperatures. There is a significant contribution from n-type electronic conductivity in highly reducing atmospheres.

 Please wait while we load your content...
Please wait while we load your content...