High-performance asymmetric supercapacitors with lithium intercalation reaction using metal oxide-based composites as electrode materials†
Abstract
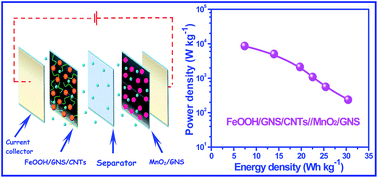
FeOOH–graphene nanosheets (GNS)–carbon nanotubes (CNTs) hybrid material was synthesized by the direct hydrolysis of Fe3+ on the surface of GNS-supported and CNTs-bridged frameworks. Interestingly, the as-obtained nanocomposite (FeOOH/GNS/CNTs) shows the lithium intercalation reaction characteristic in 1 M Li2SO4 aqueous solution as an electrode material for supercapacitor, providing high electrochemical performances. More importantly, benefiting from the nanosized FeOOH and highly coupled with conductive CNTs–GNS networks, which is beneficial to electron and electrolyte ion transport in the overall electrode, the as-fabricated asymmetric supercapacitor, using FeOOH/GNS/CNTs and MnO2/GNS as the negative electrode and positive electrode, respectively, exhibits superior energy density (30.4 W h kg−1) and good rate capability as well as excellent cycling stability (89% capacitance retention after 1000 cycles).

 Please wait while we load your content...
Please wait while we load your content...