Development of new anode composite materials for fluoride ion batteries†
Abstract
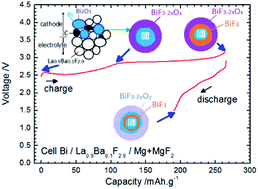
Due to their high theoretical energy density values, Fluoride Ion Batteries (FIB) are interesting alternatives to Li-ion batteries. Recently, results have been reported on the reversible charge and discharge of such systems using a solid electrolyte, various metal fluorides as cathode materials and Ce metal as the anode. The work in the present study is focused on the development of new anode materials which do not contain Li. To facilitate cell preparation and material handling, cells were prepared in the discharged state with Bi or Cu as the cathode material and CeF3, CaF2 or MgF2 as potential anode materials. The charge and discharge mechanisms were examined by detailed ex situ X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) experiments. The best cycling performances were obtained with MgF2 but prepared in the half-discharged state (i.e. mixed with Mg), thus forming a composite that could provide better interface contacts between the different reactive phases. The results showed that apart from choosing carefully the electrode active materials, it is also important to optimise the architecture of the electrodes.

 Please wait while we load your content...
Please wait while we load your content...