Peroxide defect formation in zirconate perovskites
Abstract
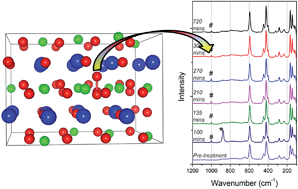
Atomic scale modelling suggests that excess oxygen can be accommodated in the group II perovskite zirconates by the formation of peroxide ion defects. This is unprecedented given the lack of charge compensating defects required for standard excess oxygen accommodation. The solution energy of O2 was predicted to be close to zero for BaZrO3, accommodating the peroxide ion defect more easily than in SrZrO3 or CaZrO3. This was experimentally examined by exposing SrZrO3 and BaZrO3 to hydrogen peroxide solution and then carrying out Raman spectroscopy measurements to look for a peak indicative of peroxide ions. A peak was observed at ∼1000 cm−1 in both compositions, suggesting the theoretically predicted peroxide ion is present.

 Please wait while we load your content...
Please wait while we load your content...