Doped h-BN monolayer as efficient noble metal-free catalysts for CO oxidation: the role of dopant and water in activity and catalytic de-poisoning†
Abstract
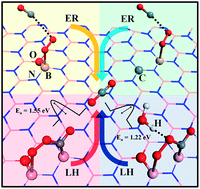
Using a first principles approach, we investigated the catalytic activity of a noble metal-free n-doped (C → B, O → N) hexagonal boron nitride (h-BN) monolayer for CO oxidation. The CO adsorption ability and hence the preferred Eiley–Rideal (ER) and Langmuir–Hinshelwood (LH) mechanism for CO oxidation is dopant-dependent: CO is chemisorbed on O-doped h-BN (OBN) while it physically interacts with the C-doped h-BN (CBN) surface. Even though both C and O doping create similar donor states below the Fermi level (Ef), O doping results in a larger bond length of O–B1 (one of the nearest B atom), out-of-plane displacement of the B1 atom, and less positive charge on the B1 atom, synergistically contributing to higher atomic activity. The presence of a pre-adsorbed O2 molecule on both types of surfaces eliminates any chances of CO poisoning of the surface, and CO oxidation prefers to proceed via the ER mechanism with a small activation barrier. The high values of Sabatier activities suggest that the doped h-BN surface is superior to Au55and Pt55nanoclusters. In case of CO oxidation by means of the LH mechanism, a stable O2⋯CO intermediate is produced, which requires a high barrier energy to break the O–O bond. However, the presence of a H2O molecule increases the activity of the catalyst and helps in catalytic CO de-poisoning.

 Please wait while we load your content...
Please wait while we load your content...