Oxygen transport kinetics of the misfit layered oxide Ca3Co4O9+δ†
Abstract
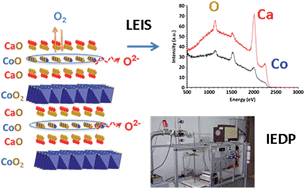
The oxygen transport kinetics of the misfit-layered cobaltite, Ca3Co4O9+δ, known for its thermoelectric properties, was investigated by combined application of 18O/16O isotope exchange and electrical conductivity relaxation techniques. Although oxygen diffusion is found to be two orders of magnitude lower than in well-investigated lanthanum nickelates, e.g., La2NiO4+δ, the mixed ionic–electronic conductor Ca3Co4O9+δ is found to exhibit fast surface exchange kinetics (k* = 1.6 × 10−7 cm s−1 at 700 °C to be compared to 1.3 × 10−7 cm s−1 for the nickelate), rendering it a promising electrode for application as an air electrode in solid oxide cells. In parallel, the chemical nature of the outermost surface of Ca3Co4O9+δ was characterized by means of Low Energy Ion Scattering (LEIS) spectroscopy. The absence of cobalt at the sample's outermost surface suggests that the Ca2CoO3−δ rock salt layers in the structure may play a key role in the oxygen exchange mechanism.

 Please wait while we load your content...
Please wait while we load your content...