Electron transfer properties of chemically reduced graphene materials with different oxygen contents
Abstract
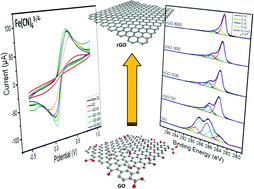
Herein, we endeavoured to tune the surface composition and hence electron transfer properties of chemically reduced graphene oxides through varying the mass of sodium borohydride (NaBH4) in the reduction step. The resulting materials were characterised via X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR), and conductivity studies. The XPS and Raman spectra show positive correlations between the mass of NaBH4 used, and C/O ratios and ID/IG ratios of the materials, respectively. The graphene materials exhibit improved conductivity with greater degrees of reduction and FTIR shows that, specifically, carbonyl groups were reduced to hydroxyl groups. The cyclic voltammetry technique was then employed to elucidate a trend between the heterogeneous electron transfer of Fe(CN)63−/4−, Ru(NH3)63+/2+, Fe3+/2+, V3+/2+, Eu3+/2+, ascorbic and uric acids, and the C/O ratios. It was found that the dependency of the heterogeneous electron transfer on the oxygen content in chemically reduced graphenes is more complex than previously thought and that multiple concurrent effects participate.

 Please wait while we load your content...
Please wait while we load your content...