Synergy of nanoconfinement and surface oxygen in recrystallization of sulfur melt in carbon nanocapsules and the related Li–S cathode properties†
Abstract
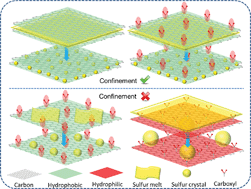
We studied the recrystallization behaviours of sulfur melt in oxygen-containing carbon nanocapsules (CNCs). The effects of the oxidizing degree and the nanoconfinement of CNCs on sulfur recrystallization were investigated. We performed weak oxidation on CNCs by firstly grafting >C-Cl3 groups via a Friedel-Craft reaction and successive hydrolysis of >C-Cl3; and the strong oxidation was conducted in nitric acid. It is found that the weak oxidation preserved the CNC structure while the strong oxidation damaged the CNC morphology. Electron microscopy, X-ray diffraction, Raman spectroscopy and X-ray absorption spectroscopy were combined to characterize the sulfur crystallites in pristine and oxidized CNCs. The results revealed that the smaller sulfur crystallites preferentially formed in integrated CNCs (preserved nanoscale hollow structure) regardless of oxygen content; while the stronger oxidation and destruction of hollow structures fostered the growth of larger sulfur crystals. These results suggest a possible approach to control the growth of sulfur in carbon by combining oxygen and nanoconfinement effects, and hopefully to tune the related electrochemical properties in Li–S battery cathodes.

 Please wait while we load your content...
Please wait while we load your content...