Facile hydrothermal synthesis of crystalline Ta2O5 nanorods, MTaO3 (M = H, Na, K, Rb) nanoparticles, and their photocatalytic behaviour†
Abstract
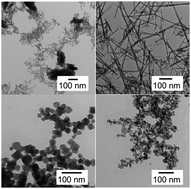
Alkali metal tantalates are of interest for applications in photocatalysis as well as in high temperature resistance or capacitor dielectric materials. We have synthesized nanosized Ta2O5 rods and MTaO3 cubes (M = Na, K, Rb) hydrothermally and demonstrate the pH dependence of the synthesis of tantalum oxide and tantalate nanoparticles. The morphologies of the nanoparticles range from particle agglomerates in acidic reaction media over rods at neutral pH to tantalate cubes in basic reaction media. Whereas there is no apparent influence of the base cation on the particle morphology, there is a pronounced effect on the particle composition. At high base concentrations cubic tantalate particles with a pyrochlore structure were formed. The pyrochlore structure allows a complete ion exchange through the tunnels in the structure by replacing the alkali metal ions by H+ while retaining the particle morphology. The as-synthesized particles show promising photocatalytic properties.

 Please wait while we load your content...
Please wait while we load your content...