Application of alkoxyamine in self-healing of epoxy
Abstract
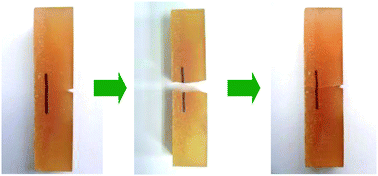
To provide epoxy with intrinsic self-healing ability, a new alkoxyamine based diol was synthesized and incorporated into an epoxy monomer, which was then compounded with traditional bisphenol A diglycidyl ether and cured by diethylenetriamine. Taking advantage of dynamic equilibrium of thermally reversible reaction of C–ON bond in alkoxyamine, cracked portions of the functionalized epoxy material can be repeatedly reconnected at certain temperature, as characterized by restoration of impact strength and visual inspection as well. Unlike the two-step self-healing approach based on reversible Diels–Alder reaction, the present one only needed one step. Owing to the steric hindrance of tertiary butyl group in the diol, onset of scission of C–ON bonds and radical recombination (i.e., healing reaction) occurred at lower temperature. Additionally, reversibility of the alkoxyamine derivatives was improved with incorporation of Si–O bonds into the epoxy chains. The present work carefully studied thermal reversibility, thermal stability, dynamic mechanical behavior and healing performance of the epoxy in relation to molecular structure. The outcomes might help to optimize the material and guide future design of novel epoxy monomers.

 Please wait while we load your content...
Please wait while we load your content...