Enhanced electrochemical performance of Li3V2(PO4)3/Ag–graphene composites as cathode materials for Li-ion batteries†
Abstract
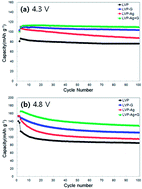
In this study, we have synthesized a Li3V2(PO4)3/Ag–graphene (LVP/Ag–G) composite using a facile sol–gel route. The physical and electrochemical properties of the LVP/Ag–G composite have been evaluated by X-ray diffraction (XRD), scanning electron microscopy (SEM), Raman spectroscopy and electrochemical measurements. In the potential range 3.0–4.3 V, the LVP/Ag–G electrode delivered a capacity of 135 mA h g−1 at a rate of 0.1 C, which is above the theoretical capacity (133 mA h g−1). Even at a high current rate of 10 C, it still exhibited a discharge capacity of 118 and 133 mA h g−1 in the potential ranges 3.0–4.3 and 3.0–4.8 V, respectively. Such a capacity value is significantly higher than previously reported works. The LVP/Ag–G composites exhibited an outstanding specific capacity, rate capability and cycling stability. These exceptional properties of the LVP/Ag–G composites were mainly attributed to the synergetic effect of the graphene and the silver particles.

 Please wait while we load your content...
Please wait while we load your content...