A DIH-based equation for separation of CO2–CH4 in metal–organic frameworks and covalent–organic materials
Abstract
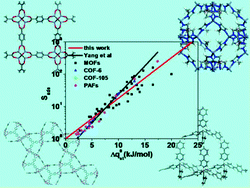
We develop a new S(DIH) equation based on the difference of isosteric heats (DIH) to calculate the selectivity for CO2 over CH4 in metal–organic frameworks (MOFs) and covalent–organic materials. Using the S(DIH) equation to predict the selectivity requires only the adsorption isotherms of pure components and the DIH of the two components. By comprehensive comparison with the GCMC data in different types of porous materials, including MOFs, ZIFs, COFs and PAFs, it is found that the new S(DIH) equation can predict with high accuracy the selectivity of different types of porous materials for CO2 over CH4 at the low pressure of p = 0–1 bar. Therefore, the new S(DIH) can serve as an efficient tool for the selectivity predictions of porous materials for CO2 over CH4 at p = 0–1 bar, especially for the cases in which experiments can measure the adsorption isotherms and adsorption heats of pure components (such as CO2, CH4, N2 and H2) because the new S(DIH) requires only the adsorption isotherms and adsorption heats of pure components as inputs. In short, the new S(DIH) equation can be considered as a valuable screening tool for obtaining an estimation about the selectivity of a porous material for a certain component of the gas mixture.

 Please wait while we load your content...
Please wait while we load your content...