Bis(fluoromalonato)borate (BFMB) anion based ionic liquid as an additive for lithium-ion battery electrolytes
Abstract
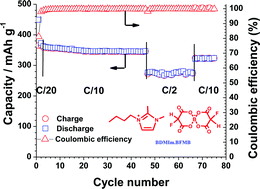
Propylene carbonate (PC) is a good solvent for lithium ion battery applications due to its low melting point and high dielectric constant. However, PC is easily intercalated into graphite causing it to exfoliate, killing its electrochemical performance. Here we report on the synthesis of a new ionic liquid electrolyte based on partially fluorinated borate anion, 1-butyl-1,2-dimethylimidazolium bis(fluoromalonato)borate (BDMIm·BFMB), which can be used as an additive in 1 M LiPF6/PC electrolyte to suppress graphite exfoliation and improve cycling performance. In addition, both PC and BDMIm·BFMB can be used synergistically as additive to 1.0 M LiPF6/methyl isopropyl sulfone (MIPS) to dramatically improve its cycling performance. It is also found that the chemistry nature of the ionic liquids has dramatic effect on their role as additive in PC based electrolyte.

 Please wait while we load your content...
Please wait while we load your content...