Facile large-scale synthesis of vertically aligned CuO nanowires on nickel foam: growth mechanism and remarkable electrochemical performance†
Abstract
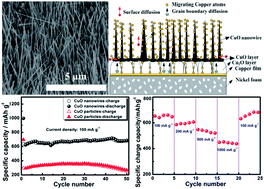
Large-scale vertically aligned single crystalline CuO nanowires grown directly on nickel foam have been successfully fabricated by facile thermal oxidation of e-beam evaporated Cu thin films in static air. A growth mechanism based on stress-driven grain-boundary diffusion associated with surface diffusion of Cu atoms/ions is proposed to explain the formation of CuO nanowires on nickel foam. The resulting CuO nanowires are directly used as binder- and conductive-agent-free electrodes for lithium ion batteries and demonstrate remarkable electrochemical performance with excellent capacity retention and high rate capability on cycling. It can deliver a stable reversible capacity of 692 mA h g−1 after 50 cycles at a current density of 100 mA g−1 and maintain a high reversible capacity of 445 mA h g−1 over 600 cycles with 95.7% capacity retention even at a high current density of 1000 mA g−1. Such superior electrochemical performance of the electrodes made by directly growing electro-active aligned CuO nanowires on conductive 3D nickel foam makes them have very promising applications in high-performance lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...