Coprecipitation fabrication and electrochemical performances of coral-like mesoporous NiO nanobars†
Abstract
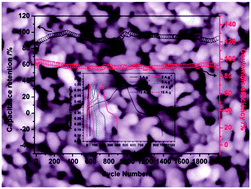
A simple and template-free coprecipitation method using Na2O2 aqueous solution as the OH− source has been developed to synthesize coral-like mesoporous NiO nanobar precursors, which were then transformed to NiO by a simple calcination procedure. The fabricated NiO nanomaterials have a relatively high specific surface area and porous structure, making them ideal candidates for pseudocapacitor applications. The pseudocapacitive properties of the as-prepared NiO electrodes have been examined by cyclic voltammetry (CV), cyclic chronopotentiometry (CP) and electrochemical impedance spectroscopy (EIS) in 6.0 M KOH solution. A high specific capacitance (SC) of 1085 F g−1 with an excellent energy density of 30.52 W h kg−1 could be obtained at a discharge current density of 1 A g−1. And the as-prepared NiO electrode also exhibits good long-term cycling stability at a high current density of 5 A g−1.

 Please wait while we load your content...
Please wait while we load your content...