Electrochemical synthesis of coaxial TiO2–Ag nanowires and their application in photocatalytic water splitting
Abstract
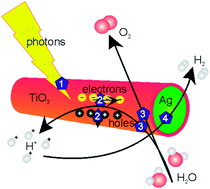
A new method for the formation of coaxial TiO2–Ag nanowires is presented, in which TiO2 nanotubes were formed by the templated electrochemically induced sol–gel method, followed by thermal annealing. The as-formed TiO2 nanotubes have been successfully filled with a Ag core using a subsequent electrodeposition step. Coaxial nanowires have a very suitable architecture for photocatalysis, solar cells or batteries due to the high contact area between the two different phases, the large outer surface area exposed to the reactant, and short electron diffusion paths. The coaxial nanowires showed a higher efficiency than empty TiO2 nanotubes and TiO2 nanotubes with attached Ag nanoparticles in photocatalytic water splitting. Coaxial TiO2–Ag nanowires formed H2 at a rate of ∼1.23 × 10−3 ± 0.3 × 10−3 mol g−1 h−1 without deactivation for at least 6 h.

 Please wait while we load your content...
Please wait while we load your content...