Investigation of metal oxide anode degradation in lithium-ion batteries via identical-location TEM†
Abstract
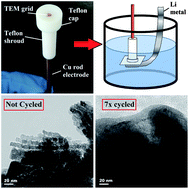
Metal oxides are one of the most promising classes of materials to replace graphite anodes in future high energy density, high power density applications for lithium-ion batteries (LIBs), including (hybrid) electric vehicles and grid-scale energy storage. Unlike graphite, which undergoes lithium ion intercalation, nearly all metal oxides (MOs) experience structural decomposition and phase separation during charge–discharge. In this work, ordered mesoporous nickel oxide (OMNiO) was synthesized and used as a representative MO to study the nanoscale structural changes that occur during charge–discharge via identical-location transmission electron microscopy (IL-TEM). We connect IL-TEM with coin cell electrochemical studies to explain the root cause for the rapid capacity fade that is common to MOs generally, and NiO specifically. We show that the electronic conductivity, in addition to MO structure, plays a crucial role in maintaining high capacity over repeated charge–discharge cycling.

 Please wait while we load your content...
Please wait while we load your content...