Novel polysilsesquioxane hybrid polymer electrolytes for lithium ion batteries†
Abstract
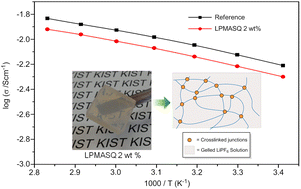
A novel inorganic–organic hybrid crosslinker was prepared through synthesis of a fully condensed, high molecular weight ladder-like poly(methacryloxypropyl)silsesquioxane (LPMASQ) in one pot with a facile, base-catalysed system. The fully condensed LPMASQ revealed good thermal (∼380 °C) and electrochemical stability (∼5.0 V) due to the absence of uncondensed silanol groups. LPMASQ also revealed good solubility in various organic solvents and fully gelated 1 M LiPF6 in ethyl carbonate–diethyl carbonate (EC–DEC, 3/7, v/v) electrolyte solution through fast thermal and photocuring even at a very low concentration of 2 wt%. These observations were attributed to the polymeric nature of LPMASQ containing over one hundred methacryl moieties on the rigid double-stranded siloxane backbone. To the best of our knowledge, formation of a gel polymer electrolyte with 2 wt% gelator is the smallest gelation concentration that has ever been reported. This leads to high ionic conductivity (∼6.0 mS cm−1), excellent Coulombic efficiency and battery cell performance, comparable with those of the neat liquid electrolyte. The small crosslinker content, thermal and electrochemical stability, fast thermal and photocuring and facile processing of the LPMASQ based GPEs, as well as excellent Li battery cell performances strongly hold great promise for future industrial battery applications.

 Please wait while we load your content...
Please wait while we load your content...