A fluoride-doped PEG matrix as an electrolyte for anion transportation in a room-temperature fluoride ion battery†
Abstract
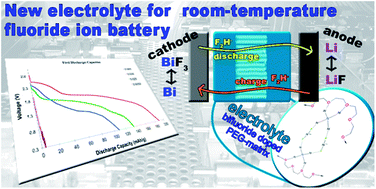
Using liquid electrolytes could increase the efficiencies of fluoride ion batteries. Such an electrolyte should demonstrate good fluoride transfer, be soluble in common solvents, and exhibit stability against decomposition. We have developed a new electrolyte system, which is based on an ammonium bifluoride-doped polyethylene glycol matrix. This compound shows good stability to thermal decomposition up to about 340 °C. The good ionic conductivity at low concentration (0.02 M) could make this compound an appropriate electrolyte. We tested our fluoride-doped PEG matrix in test cells and the first discharge capacity was found to range around 189 mA h g−1 (with respect to the active mass of the cathode) under nonoptimized conditions. Furthermore, we also examined the impact of lithium ions that may dissolve from the anode into the electrolyte.

 Please wait while we load your content...
Please wait while we load your content...