Is Li4Ti5O12 a solid-electrolyte-interphase-free electrode material in Li-ion batteries? Reactivity between the Li4Ti5O12 electrode and electrolyte†
Abstract
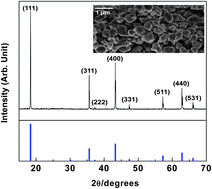
Does the Li4Ti5O12 electrode need a carbon additive in lithium-ion batteries? We answered this question in our previous work by showing that the partially reduced Ti4+ on the surface of Li4Ti5O12 could provide enough electronic conductivity to initiate the electrochemical process. In this work, we discuss the generally accepted fact that a solid electrolyte interphase (SEI) is hardly formed on the surface of the Li4Ti5O12 electrode owing to its high redox potential by using the carbon-free Li4Ti5O12 electrode to exclude the influences of carbon. In contrast to the previous argument, Li4Ti5O12 was found to have certain reactivity towards electrolytes at room and high temperature (60 °C). Moreover, in the presence of carbon in the electrode (i.e. the conventional electrode formulation), the reactivity of the electrode towards an electrolyte was significantly increased at high temperatures. These results were discussed based on surface analyses and electrode morphology observation before and after cycling, and correlated with the electrochemical performance.

 Please wait while we load your content...
Please wait while we load your content...