The role of ions and reaction sites for electrochemical reversible charge cycling in mesoporous nickel hydroxides†
Abstract
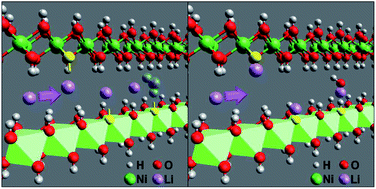
Layered nickel hydroxide thin films with mesoporous structure were prepared on ITO substrates by a facile chemical bath deposition method. The electrochemical properties of the nickel hydroxide/oxyhydroxide films were investigated in both potassium hydroxide and lithium perchlorate in propylene carbonate electrolytes. We show that the high reversible charge cycling capability of the material is enabled by the redox reaction involving hydroxyl ions, especially in the oxidative cycle. Li ion reversible charge cycling requires available reaction or adsorption sites that can be provided by sub-stoichiometry or defective films. Raising the defect concentration, the cyclic reversibility is shown to increase by ∼5 times. The effects of intercalated water in the interlayers are also discussed. We show that the presence of water in the interlayers can lead to a passivating reaction during the charge cycling and also cause optical efficiency losses through unwanted charge trapping.

 Please wait while we load your content...
Please wait while we load your content...