Solventless synthesis of an iron-oxide/graphene nanocomposite and its application as an anode in high-rate Li-ion batteries†
Abstract
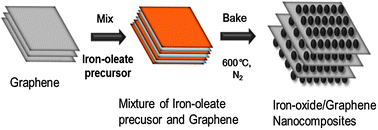
An iron-oxide/graphene nanocomposite was directly synthesized from an iron–oleate precursor intercalated between graphene layers via a solventless thermal decomposition method. In the nanocomposite, highly monodisperse γ-Fe2O3 nanoparticles were in close contact with graphene, and they acted as mutual spacers in the nanocomposite to prevent aggregation of the nanoparticles and restacking of the graphene layers. The iron-oxide/graphene nanocomposite shows outstanding electrochemical performance, including high reversible charge/discharge capacity, good cycling stability, and remarkable high-rate-performance (500 mA h g−1 at 5000 mA g−1) when it was employed as a lithium ion battery anode. This nanocomposite can be a potentially valuable candidate anode material for high-rate Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...