Synthesis and electrochemical performance of rod-like spinel LiMn2O4 coated by Li–Al–Si–O solid electrolyte
Abstract
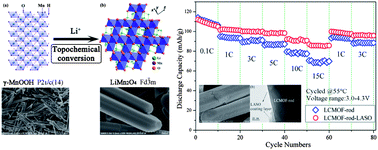
Li1.05Co0.1Mn1.9O3.95F0.05 rods with a spinel structure were synthesized by a topochemical conversion route, and a Li–Al–Si–O (LASO) glassy solid electrolyte was used to modify the surface of the cathode material. The structure and electrochemical properties were investigated using X-ray diffraction, scanning electron microscope, transmission electron microscope and electrochemical tests. XRD, SEM and TEM observations show that Li1.05Co0.1Mn1.9O3.95F0.05 materials have a single crystal of cubic spinel structure and exhibit a rod-like morphology with a diameter of 400–500 nm. The electrochemical results indicate that LASO-coated Li1.05Co0.1Mn1.9O3.95F0.05 rods exhibit an excellent rate capability and cycle stability at elevated temperatures and high rates. The initial discharge capacity of the LCMOF-rod-LASO sample is 93.9 mA h g−1 under 10 C at 55 °C, and the capacity retention ratio is higher than 96% after 200 cycles. Even under 15 C at 55 °C, the cathode material shows a high capacity of 87 mA h g−1. The cyclic voltammograms and impedance spectra results reveal that the LASO coating enhances the kinetics of the lithium-ion diffusion through the surface layer and the charge transfer reaction, improving the electrochemical activity of the cathode. Thus, the LASO-coated Li1.05Co0.1Mn1.9O3.95F0.05 rods have shown potential for high power applications in lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...