Facile green synthesis of nickel nanostructures using natural polyol and morphology dependent dye adsorption properties†
Abstract
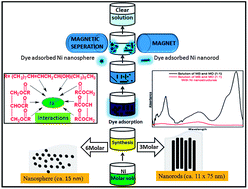
Ni nanostructures were synthesized using only castor oil (CSO), a natural polyol through green chemistry. CSO acts as multifunctional reagent (solvent, reducing agent, template, surface modifier) for the synthesis of the Ni nanostructures and imparts dye adsorption ability. The morphology of the nanostructures was found to be influenced by variations in the precursor concentration. A three molar concentration resulted in the formation of nanorods (ca. 11 × 75 nm) due to a higher nucleation rate as compared to growth rate, while a six molar concentration produces nanospheres (ca. 15 nm) due to a higher growth rate. The progress of the reaction, synthesis mechanism and surface functionalization of the Ni nanostructures were analyzed by Fourier transform infrared (FT-IR) spectroscopy. The interaction of Ni2+ with electron rich sites in the CSO resulted in the reduction of Ni2+ to Ni(0). The cubic lattice of the synthesized nanostructures, phase and preferred growth direction of the nanorods along the [200] plane were determined by correlation of X-ray diffraction (XRD) results with that of transmission electron microscopy (TEM) analysis. The adsorption ability of the magnetic Ni nanostructures was analyzed using parameters such as the adsorption kinetics (rate and order of adsorption), maximum adsorption capacity (250 mg g−1 (nanospheres) and 142 mg g−1 (nanorods)), effect of initial concentration of dye/adsorbent and contact time. The Langmuir and Freundlich adsorption isotherms were employed for an understanding of the nature (chemisorption or physisorption) of the dye adsorption. The magnetic nature of the adsorbent helps in the fast, economical separation of an adsorbed particle in comparison to non-magnetic dye adsorbents. The better and favorable adsorption (RL value) of the dye on the synthesized Ni nanostructures, makes them a potential adsorbent for the removal of toxic dyes from industrial effluents.

 Please wait while we load your content...
Please wait while we load your content...