Synthesis of well-crystallized Li4Ti5O12 nanoplates for lithium-ion batteries with outstanding rate capability and cycling stability
Abstract
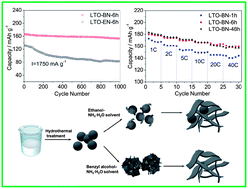
As a lithium-intercalation material, high crystallinity is important for Li4Ti5O12 to achieve good capacity and cycling stability, while a large surface area and a short lithium diffusion distance are critical to increase rate capacity. In this study, well-crystallized Li4Ti5O12 nanoplates with outstanding electrochemical performance were facially prepared through a two-step hydrothermal preparation with benzyl alcohol–NH3·H2O (BN) as the solvent and a subsequent intermediate-temperature calcination at 500 °C for 2 h in air. To support the superiority of benzyl alcohol–NH3·H2O (BN) for hydrothermal synthesis, ethanol–NH3·H2O (EN) was also comparatively studied as solvent. In addition, different hydrothermal reaction times were tried to locate the optimal reaction time. The nature of as-prepared Li4Ti5O12–BN (LTO–BN) and Li4Ti5O12–EN (LTO–EN) was characterized by XRD, N2 adsorption/desorption tests, SEM, TEM and TGA-DSC. Compared with EN, the BN hydrothermal solvent facilitated the formation of nanosheet-Li4Ti5O12 with wall thicknesses of 8–15 nm and better crystallization. After a 6 h hydrothermal reaction at 180 °C and subsequent calcination, well-crystallized Li4Ti5O12–BN nanoplates were produced, which demonstrate a superior discharge capacity of 160 mA h g−1, even at 40 C, maintaining a capacity of 88.8% compared with that at 1 C. The nanoplates also exhibited excellent cycling stability, retaining a discharge capacity of 153 mA h g−1 after 1000 charge–discharge cycles at 10 C.

 Please wait while we load your content...
Please wait while we load your content...