The effects of pyridine on the structure of B-COFs and the underlying mechanism†
Abstract
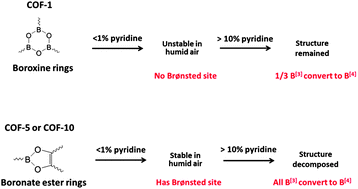
Previous work demonstrated the ability of a trace amount of pyridine to stabilize Covalent Organic Frameworks (COF)-5 and -10 in humid air. Pyridine was found to form a mixture of Lewis and Brønsted B[4] Py–B complexes in addition to the un-complexed B[3] sites in the framework structures. Further research has shown that higher doses of pyridine convert all remaining B[3] in COF-5/-10 to Lewis B[4] and bring about the total and irreversible structural decomposition of COF-5 and COF-10. The results suggest that the accumulated strain in the five-member rings of COF-5/-10 resulting from the formation of tetrahedrally-distorted B[4] sites at high pyridine loadings, may explain the decomposition of these structures. Alternatively, COF-1 is unstable to exposure to humid air at all pyridine loadings tried, but is not unstable to high doses of pyridine. Whereas the same tetrahedrally-distorted B[4] sites are formed in COF-1, in this case the six-membered B3O3 ring can accommodate the accumulated ring strain and retain an ordered structure. A thorough solid state NMR and molecular dynamics investigation has led to a new proposed stabilization mechanism in humid air based on the formation of Brønsted B[4].

 Please wait while we load your content...
Please wait while we load your content...