Aqueous Li+/Al3+ alkaline solution for CO2 capture and the massive Li–Al–CO3 hydrotalcite precipitation during the interaction between CO2 gas and the Li+/Al3+ aqueous solution
Abstract
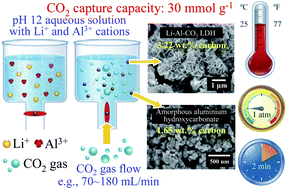
The concentrations of Al3+ and Li+ in an aqueous alkali affect the ability of the solution to capture CO2. When CO2 gas was forced into such a solution, CO2 was captured via the formation of a carbonate-containing compound. The carbonate-containing compound was Li–Al–CO3 hydrotalcite (Li–Al–CO3 LDH) or amorphous aluminium hydroxycarbonate. The former compound contained 2.84–3.22 wt% carbon, while the latter contained less carbon (1.52–1.97 wt%). The Li–Al–CO3 LDH was porous with nanosized pores and had a BET surface area of 114.8–161.8 m2 g−1. The injection of a relatively low CO2 gas flow rate into the Al3+- and Li+-containing aqueous solution favoured the production of Li–Al–CO3 LDH. For example, forcing CO2 gas into the aqueous solution at a flow rate of 70 mL min−1 produced Li–Al–CO3 LDH. Injecting CO2 at a higher flow rate (120 mL min−1) into the same solution produced amorphous aluminium hydroxycarbonate. A CO2 capture capacity (mmol per gram AlLi IMC) of up to ∼30 mmol g−1 (1.30 kg CO2 per kg of AlLi) was achieved in 120 s. The fall in the pH of the alkaline solution during CO2 bubbling was the critical factor that determined whether the final product was Li–Al–CO3 LDH or amorphous aluminum hydroxycarbonate.

 Please wait while we load your content...
Please wait while we load your content...