Reversible hydrogen sorption in NaBH4 at lower temperatures
Abstract
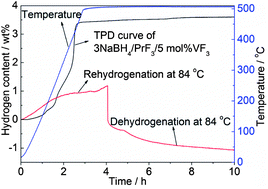
In the present study, a new hydrogen storage system, being able to reversibly absorb/desorb hydrogen at fairly low temperatures, was developed based on a 3NaBH4–PrF3 composite. It is shown that 3 wt% of reversible hydrogen sorption can be achieved in the 3NaBH4–PrF3 composite at 400 °C with fast kinetics. After the addition of 5 mol% VF3, the dehydrogenation kinetics of the 3NaBH4–PrF3 composite can be significantly improved. The onset dehydriding temperature is lowered down to 46 °C in vacuum, and the dehydrogenation finishes in 2 min at 400 °C. Both the dehydrogenation enthalpy and activation energy of 3NaBH4–PrF3 can be lowered down through the addition of VF3. In particular, the dehydrogenation products of the 3NaBH4–PrF3–5 mol% VF3 composite can be rehydrogenated at a temperature as low as 48 °C with the regeneration of NaBH4. At 84 °C, a reversible hydrogen sorption of about 1.2 wt% can be achieved in the 3NaBH4–PrF3–5 mol% VF3 composite. The improvement in hydrogen sorption properties can be mainly attributed to the formation of the VB2 phase during dehydrogenation as an efficient catalyst, which maintains well its catalytic effect in the re-/dehydrogenation cycles. Based on a series of controlled experiments and phase analyses, the de-/rehydrogenation mechanisms of the 3NaBH4–PrF3 composite without and with VF3 addition are proposed and discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...